How to accurately measure the chemical oxygen demand parameter for the samples containing a very high chloride concentration
What is Chemical Oxygen Demand (COD)?
COD is defined as the amount of a specific oxidant (expressed in its oxygen equivalence) that reacts with a sample with organic substances under controlled conditions-Dichromate ion (Cr2O72-) is reduced to its chromic ion (Cr3+).
COD is an indirect measure of the amount of organics in a water sample. Like the BOD test, oxygen is used to oxidize the organics to carbon dioxide and water. However, instead of free dissolved oxygen, chemically bound oxygen in potassium dichromate (K2Cr2O7) is used to oxidize the organics.
COD Reaction:
CnHaObNc + d Cr2O72- + (8d+c) H+ → Δ → nCO2 + (a+8d-3c)/ 2H2O + c NH4+ + 2 dCr3+
here, NH3 converts to NH4+ due to acidic medium.
The oxidation of organics results in the reduction of Cr6+ to Cr3+. The amount of dichromate is used is proportional to the amount of organics present. Likewise, the amount of Cr3+ ion present is proportional to the amount of organics digested.
Organics + K2Cr2O7 (Cr6+) (Orange/yellow) → Cr3+ (Green)

More oxidant consumed = High levels of organics; Less oxidant consumed = Low levels of organics
What is Chemical Oxygen Demand (COD) method?
The COD method determines the quantity of oxygen required to oxidize the organic matter present in wastewater when subjected to oxidation by a strong chemical oxidant, temperature, and time.
COD is a two-step method:
1st Step: Digestion
2nd Step: Determination by titrimetric method or colorimetric method
For the digestion to occur, the reaction needs: acid, heat, and a catalyst.
Acid: Concentrated sulfuric acid (H2SO4) which is the primary digestion catalyst.
Silver sulfate (AgSO4): this act as a secondary catalyst which assists in oxidization of straight-chain polycarbons (i.e, diesel fuel and motor oil).
Note: The silver must be soluble and it precipitate if chlorides are present in the sample. To prevent silver precipitation, mercury is added to the reagents in the tube. The mercury removes the chloride interference.
Heat: the heat is provided by the digester (reactor), which is typically set at 150 °C. The sample is refluxed (digested) for 2 hours. During the 2 hours, the organics are oxidized with the hexavalent dichromate ion (Cr2O72-) found in potassium dichromate (K2Cr2O7).
Colorimetric Analysis:
The dichromate ion is visible at 420 nm, and the Cr3+ ion around 600-620 nm. A spectrophotometer sends the correct wavelength through the sample cell to a detector that measure transmittance.
Low Range (LR) COD – measures decrease in oxidant (Cr2O72-) or orange/yellow color at 420nm.
High Range (HR) COD – measures increase in Cr3+ or green color at 620nm.
COD methods commonly used in the Water & Wastewater Sample Analysis:
- ISO 15705:2002 Determination of the COD Index – Small-scale Sealed-Tube method
- GB 11914 and Modified GB 11914 Method
- EPA Approved COD Methods for NPDES Reporting
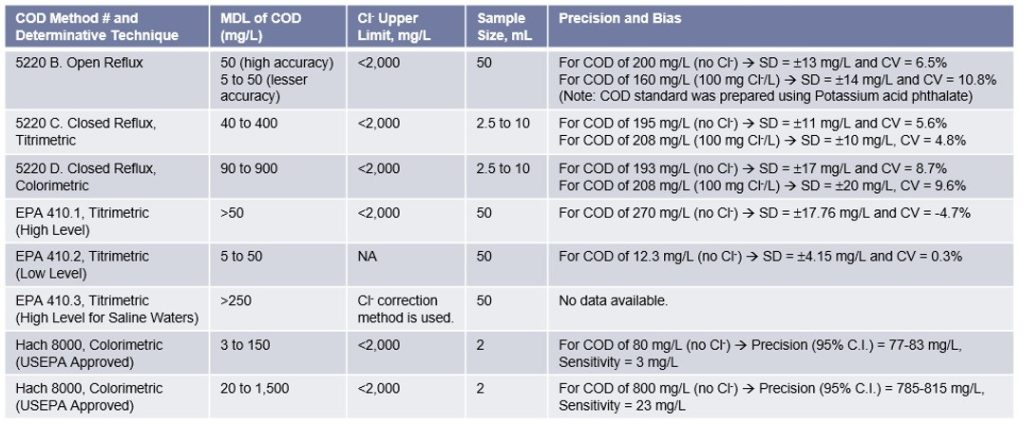
Significance of Removal of Chloride Interferences for accurately measuring the COD concentration:
Chloride concentration is the primary interference when determining the COD concentration. Each COD vial (used in COD measurement using the spectrophotometers) contains mercuric sulfate that eliminates chloride interference up to 2,000 mg/L. Samples with higher chloride concentrations are typically diluted.
Method of Preventing Chloride Interference
Several methods have been reported for preventing interference by chloride ions in the COD test.
- The silver(I) sulfate is added after an initial reflux period. In this process, chloride reacts with AgSO4 precipitating AgCl reducing or inhibiting the Silver catalytic activity (reaction 1). Chlorides in dichromate gets oxidized to chlorine gas producing erroneous high COD levels (reaction 2). This process allows for complete oxidation of chloride but does not prevent any interactions between the chloride/chlorine and other components of the sample.
Ag+ + Cl- → AgCl (reaction 1)
6Cl- + Cr2O72- (aq) + 14H+ (aq) → 3Cl2 + 2Cr3+ (aq) + 7 H2O (reaction 2)
- The chloride is precipitated with silver(I) and the silver chloride is removed. However, this method suffers from a great disadvantage in that organic material may be adsorbed on, or included in the precipitate.
- Mercury(II) sulfate is added, which forms the soluble but largely undissociated mercury(II) chloride, thus removing almost all of the free chloride from the solution. This is the procedure prescribed in most standard methods. However, even with a large excess of mercury(II), the oxidation of chloride is not completely prevented.
- The conditions of the test are altered so that oxidation of chloride is inhibited: the oxidation potential of the system is lowered by decreasing the dichromate concentration and the acid concentration. However, this will also cause a decrease in the amount of organic material oxidized.
- Silver(I) sulfate is added and the sample oxidized in a closed container. Loss of chlorine is prevented and the increase in the chlorine concentration in the solution suppresses the chloride oxidation. However, this will not prevent chlorination of organic compounds by the chlorine in solution, nor will it prevent the oxidation of ammonium ions by chlorine.
Basic requirements for any method are:
- The chloride concentration must be reduced to a level at which the error caused by the interference is insignificant.
- The organic content of the sample must not be altered to a significant extent (i.e, there must be no increase or decrease in COD).
- The method should be suited for routine use (i.e, it should be relatively inexpensive and it should not involve a great deal of manipulation or complicated apparatus).
Tricks for Accurately measuring the COD concentration from a sample containing a high to very high chlorides concentration and a sample containing a very low chlorides concentration:
- For COD samples, use a Hach 8000 LR (Low Range) method for effluent samples which contains higher chloride concentrations and for influent samples a Hach 8000 HR (High Range) method.
- For samples with chloride concentrations greater than 2,000 mg/L, dilute samples to reduce the chloride concentration to below 1,000 mg/L.
- For saline water samples, the method of measurement shall be approximately 5 mg COD/L with 150 mg/L vials. When 1,500 mg/L vials are used, the increase of Cr3+ is determined in the 600 nm region. When the 150 mg/L vials are used, the decrease of Cr2O72- is determined in the 400 nm region.
